Therapeutic Areas
Infectious Disease Testing and Monitoring
Viracor has extensive experience performing pathogen load monitoring, antiviral resistance assessment, nucleic acid sequencing and other pathogen characterizations for clinical trials.
With a wide range of viral and bacterial targets, you have access to a comprehensive menu of real-time PCR and sequencing assays for pathogen detection and monitoring. Viracor’s multiple-target, dual gene assays, an approach we adopted early in the transition to molecular diagnostics, are designed to eliminate false negative results or under-quantification.
COVID-19
As experts in infectious disease assays, we offer a broad array of testing solutions for biomarker detection, immunogenicity, and other safety and efficacy assessments to support anti-viral and vaccine candidate clinical programs.
We also offer validated molecular and serology tests for SARS-CoV-2, along and other bioassay solutions that are helping our clients COVID-19 vaccine and anti-viral therapy development efforts.
In fact, our SARS-CoV-2 RT-PCR assay was reported by the US FDA to be the most sensitive assay (180 NDU/mL) of the 117 evaluated.*
We also offer SARS-Cov-2 spike gene and whole genome sequencing (WGS) assays on multiple sample types, to fit your COVD-19 vaccine and anti-viral development needs.
Coronavirus (COVID-19) Testing Portfolio:
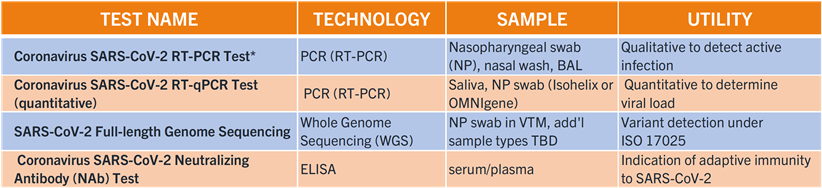
*The Eurofins-Viracor SARS-CoV-2 RT-PCR diagnostic test, was ranked as the most sensitive out of more than 117 kits evaluated by the US FDA SARS-CoV-2 Reference Panel (https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data#table2c). Furthermore, this RT-PCR test maintains very high sensitivity in the detection of variants such as B.1.1.7 and B.1.351.
Learn more about our coronavirus testing portfolio in our COVID-19 Testing for Clinical Trials brochure.
Eurofins Viracor COVID-19 RT-PCR Test Coverage for New Variants/Strains of SARS-CoV-2
Variants of the SARS Cov-2 virus are becoming more prevalent in parts of the United States, and a potential consequence of emerging variants is the ability to evade detection by viral diagnostic tests. Eurofins Viracor, as a leading supplier of COVID-19 testing solutions, chose an assay design that anticipates genetic variation, and we maintain vigilant surveillance efforts for new variants.
Eurofins Viracor SARS CoV-2 RT-qPCR Assay Design
Our assay employs a very similar approach as taken by the US CDC. Two independent assays target different regions of the nucleocapsid gene (N gene). This dual target design was chosen to mitigate the risk of loss of sensitivity (under-quantitation and false-negativity reporting); and helps provide confidence that our tests can identify COVID-19 positive subject samples even when mutations in the genome arise. The odds of both assays sustaining significant reduction in performance due to a variant’s mutation profile are very low.
OMICRON STRAIN COVERAGE UPDATE:
The following information is specific to the Eurofins Viracor COVID-19 RT-PCR Test Coverage of the Omicron Strain (B.1.1.529) of the SARS-CoV-2 virus, as of 11/29/2021:
To ensure that our assay continues to provide reliable results, Eurofins Viracor monitors the variant situation closely, utilizing a custom bioinformatics algorithm. We regularly query the GISAID1 database for all new entries of N gene sequences. The GISAID database has 125 accessions for the Omicron strain (B.1.1.529).
Our SARS-CoV-2 assay has two independent assays (N1 and N2) running as a multiplex in the reaction. This dual target design was chosen to mitigate the risk of loss of sensitivity; and helps provide confidence that our tests can identify COVID-19 positive subject samples even when mutations in the genome arise.
Assay N1 matches the Omicron strain (B.1.1.529) 100% for the reverse primer and the probe for the available 125 entries, meaning the N1 will fully detect this strain. Assay N2 matches the Omicron strain (B.1.1.529) 100% for the reverse primer and the probe, but has a 3 base mismatch on the 5’ end of the forward primer. However, we have tested the impact of this 3 base mismatch previously (as part of our investigation into the Alpha variant) and found no significant impact on detection or the Ct value generated by the N2 assay.
Therefore, as of November 29, 2021, our SARS-CoV-2 RT-qPCR assay is predicted to detect all known variants, including the Omicron strain.
*The primary global repository for SARS CoV-2 sequencing results.
All COVID-19 assays developed and performed to support our client's pharmaceutical development programs, are for research only,
Viracor offers clients a wealth of infectious disease testing options for clinical studies, including:
- Custom development, validation and optimization of qPCR and genotyping assays for wide range of pathogens
- Multiple-target, dual gene assays, designed to eliminate risk of false negatives or under-quantified viral loads.
- Wide array of specimen types validated, and rapid TAT
Additional Chronic Viral Infection Testing:
- Human Immunodeficiency virus (HIV)
- Hepatitis B virus (HBV)
- Hepatitis C virus (HCV)
- Human T-lymphotropic virus (HTLV)
- Cytomegalovirus (CMV)
- Epstein-Barr virus (EBV)
- Adenovirus (ADV)
- Human herpesvirus (HHV-6, HHV-7, HHV-8)
- BK virus (BKV)
- John Cunningham (JC) virus


