Prioritize Patient Health with TTV Viral Load Monitoring
TTV (Torque Teno Virus) viral load monitoring is revolutionizing clinical diagnostics by providing crucial insights into patient health, especially for those with compromised immune systems, such as solid organ transplant recipients. This advanced diagnostic tool can be essential for assessing both infection and rejection risks in transplant patients, ensuring optimal care and treatment. Utilizing the stable analyte of viral DNA and the highly precise qPCR analysis method, TTV viral load monitoring can offer accuracy and a broad dynamic range.
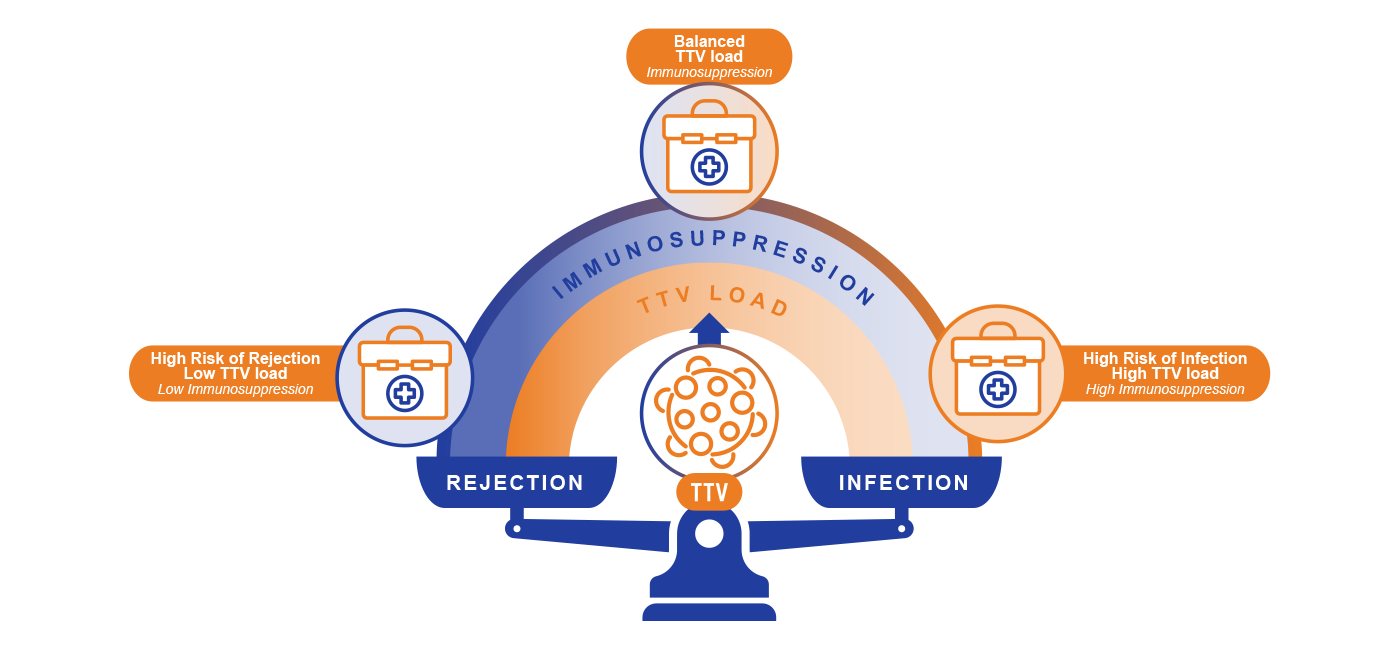
TTV viral load in plasma indicates the intensity of host immunosuppression and is associated with the risk of allograft rejection and infectious disease.
- Higher TTV viral load is associated with a higher risk of infection, lower risk of Rejection
- Lower TTV viral load is associated with a higher risk of rejection, lower risk of infection
Contact us to learn more about the TTV assay
First in Testing Innovation
Safeguarding the Health of Solid Organ Transplant Recipients
TTV - Torque Teno Virus
Risk Stratification for Solid Organ Transplant Recipients
Improved and standardized methods are needed to monitor the net state of immunosuppression in solid organ transplant recipients.
Torque Teno Virus (TTV) is a non-pathogenic virus that has been widely studied as a biomarker of the net state of immunosuppression.
- Increases in TTV viral load have been widely correlated with a decreased risk of allograft rejection and an increased risk of infections.
- For each 10-fold increase in TTV load, rejection risk decreases by 25% and infection risk increases by 4%
- The proposed TTV target range for balanced immunosuppression is 106 to 108 copies/mL.
References:
Doberer K, Schiemann M, Strassl R, Haupenthal F, Dermuth F, Görzer I, Eskandary F, Reindl-Schwaighofer R, Kikić Ž, Puchhammer-Stöckl E, Böhmig GA, Bond G. Torque teno virus for risk stratification of graft rejection and infection in kidney transplant recipients-A prospective observational trial. Am J Transplant. 2020 Aug;20(8):2081-2090. doi: 10.1111/ajt.15810. Epub 2020 Mar 8. PMID: 32034850; PMCID: PMC7496119.
Irene Görzer, Frederik Haupenthal, Fabrizio Maggi, Fanny Gelas, Dorian Kulifaj, Ludovic Brossault, Elisabeth Puchhammer-Stöckl, Gregor Bond, Validation of plasma Torque Teno viral load applying a CE-certified PCR for risk stratification of rejection and infection post kidney transplantation, Journal of Clinical Virology, Volume 158, 2023, 105348, ISSN 1386-6532,
van Rijn AL, Roos R, Dekker FW, Rotmans JI, Feltkamp M. Torque teno virus load as marker of rejection and infection in solid organ transplantation - A systematic review and meta-analysis. Rev Med Virol. 2023 Jan;33(1):e2393. doi: 10.1002/rmv.2393. PMID: 36056751; PMCID: PMC10078304.


