Innovations in Immunity
The ExPeCT anti-CD19 CAR T assay is an intervention tool which allows the Clinical team to provide patient care by directly monitoring the effectiveness of CAR T-cell therapy
-
Assess current immune response
-
Expansion and persistence of CAR T-cells
-
Evaluate treatment decisions
33285 - ExPeCT™ CAR T Chimeric Antigen Receptor Persistence and Expansion Test CD19-FMC63
ExPeCT anti-CD19 (FMC63) CAR T
ExPeCT anti-CD19 (FMC63) CAR T qPCR will allow you to personalize patient care by directly following the expansion and persistence of CAR-T cell therapy.
Longitudinally monitoring CAR-T expansion / persistence through our qPCR assay gives you real-time data of the CAR-T therapy.
This qPCR is meant to be an adjunctive measure to your current protocol providing further clinical insights into your patients.

personalized patient care
The Advantages of ExPeCT anti-CD19 (FMC63) CAR T


For Clinical Staff
- Provides interventional data for therapeutic treatment
- Quantifiable, numerical values for accurate measurement
- Ability to longitudinally monitor status of CAR T-cells

For Lab Staff
- Collection does not require additional staff or training
- Simple blood draw, no extra processing to isolate PBMC
- Not a "live cell" assay
Hybrid-Capture PCR: Precision Monitoring for ExPeCT™ (obe-cel) CAR T-Cell Therapy
Clinicians need more than indirect markers - they need certainty. Our hybrid-capture PCR assay delivers precise, real-time insights into CAR T-cell (obe-cel) engraftment and persistence, empowering you to predict outcomes, intervene early, and optimize patient care. Unlike B-cell aplasia monitoring, this assay provides direct, quantitative evidence of therapeutic activity, ensuring your patients stay on the path to durable remission.
Obe-cel, also marketed as Aucatzyl,® is a second-generation anti-CD19 chimeric antigen receptor (CAR) T-cell therapy used to treat adults with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). It was developed with a unique "fast-off" mechanism designed to reduce toxicity, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), compared to other CAR T-cell therapies. Obe-cel has received approval from regulatory bodies including the U.S. Food and Drug Administration (FDA) and the European Commission.
What This Assay Does
- Direct Detection of obe-cel CAR transgene sequences in patient samples.
- Quantifies Engraftment & Persistence to track therapy effectiveness over time.
- Early Identification of poor expansion or loss of CAR T-cells before clinical relapse.
Why It’s Better Than B-Cell Aplasia Monitoring
- Direct vs Indirect: Detects CAR T-cells themselves, not just CD19 depletion.
- Higher Specificity: Confirms therapy activity even when B-cell counts normalize.
- Quantitative & Longitudinal: Provides precise copy numbers of data for months or years.
- Early Warning: Identifies engraftment issues before clinical markers appear.
Features & Benefits
- High Sensitivity & Specificity
Detects low-level CAR T presence for confident clinical decisions. - Actionable Insights
Guides retreatment, bridging therapy, or transplant planning. - Standardized Workflow
PCR-based method fits seamlessly into molecular labs. - Supports Compliance & Research
Generates robust pharmacokinetic data for trials and post-market surveillance.

When it comes to CAR T-cell therapy for pediatric patients, several factors need to be considered during laboratory testing to ensure optimal treatment outcomes
These considerations include;
- the unique biological characteristics of pediatric patients
- the specific types of cancers prevalent in this population
- the potential impact of prior treatments
interventional data providing a reliable means of longitudinal monitoring anti-CD19 CAR-T cell expansion and/or persistence
How It Works
ExPeCT anti-CD19 (FMC63) CAR T
Eurofins Viracor has developed and validated a multiplexed quantitative real-time PCR (qPCR) assay targeting FMC63 for the longitudinal monitoring of CD19-directed CAR T-cell therapy.
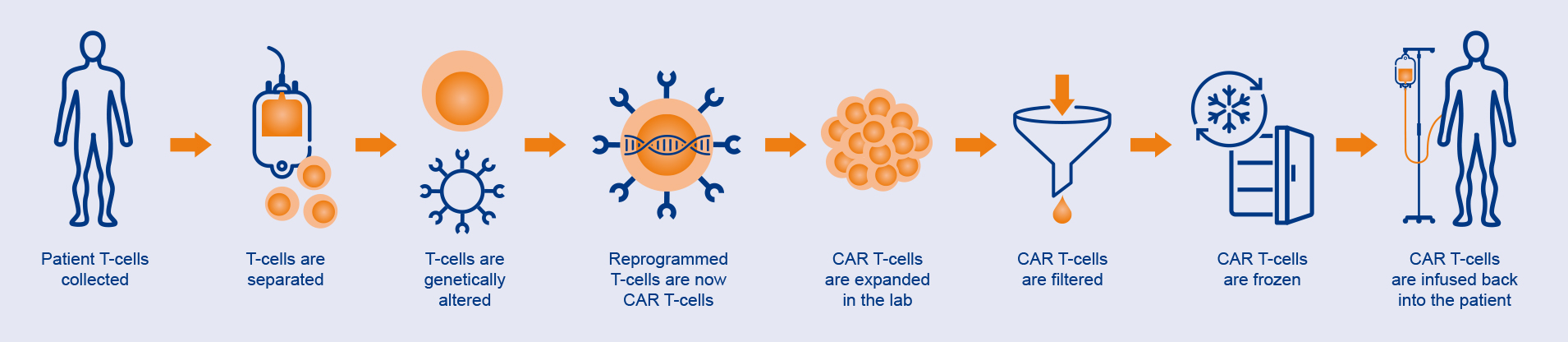
How CAR T-cells are manufactured
Development of a Multiplexed Quantitative PCR Assay for CD19-Directed CAR T-Cell Persistence Monitoring
Chimeric antigen receptor (CAR) T-cell therapy has transformed hematological cancer treatment, with six therapies now approved by the FDA. Four of these therapies (tisa cel, axi cel, brexu cel, and liso cel) target CD19 using single chain variable fragments (scFvs) derived from the heavy and light chain variable (V H and V L ) regions of the monoclonal antibody FMC63.
The primary objective of this study was to evaluate the ability of FMC63 scFv specific real time PCR (qPCR) to detect and quantify FMC63 DNA in whole blood specimens. This assay is intended for the quantitative detection of FMC63 DNA from CAR T-cells in whole blood specimens for the purpose of monitoring CAR T-cell engraftment, expansion, and persistence in individuals receiving CD19 directed CAR T-cell therapy.
Here we describe the development, validation and performance characteristics of a multiplexed, quantitative qPCR assay targeting FMC63 for the monitoring of CD19 directed CAR T-cell therapy.


Listen to Our Latest CAR T Podcast
For Hematology/Oncology patients the ExPeCT anti-CD19 (FMC63) CAR T assay provides interventional data which may provide information on the expansion and persistence of the genetically modified T-cells used as therapeutic treatment for specific lymphomas and leukemias (Chimeric Antigen Receptor T-cell therapy or CAR T). Eurofins Viracor has developed and validated a multiplexed quantitative real-time PCR (qPCR) assay targeting FMC63 for the longitudinal monitoring of CD19-directed CAR T-cell therapy.



