Exploring the History and Future of CAR T-cell Therapy and Eurofins Viracor Testing

What is CAR T-cell therapy?
CAR T-cell therapy is a promising new approach to treating cancer. CAR T-cell therapy stands for chimeric antigen receptor T-cell therapy. It involves taking a patient's T-cells, a type of immune cell, and genetically modifying those T-cells to recognize and attack cancer cells. This is done by adding a chimeric antigen receptor (CAR) to the T-cells. This therapy has shown remarkable success in treating certain types of blood cancers, such as leukemia and lymphoma, and is rapidly advancing in clinical trials for other types of cancer. The successes with chimeric antigen receptor (CAR) T cell therapy involving patients with pre-B cell acute lymphoblastic leukemia (ALL) or B cell lymphomas have revolutionized anti-cancer therapy, thereby providing a potentially curative option for patients who are refractory to standard treatments. In this post, we will explore the science behind CAR T-cell therapy, its history, and its potential as a game-changing treatment for cancer.
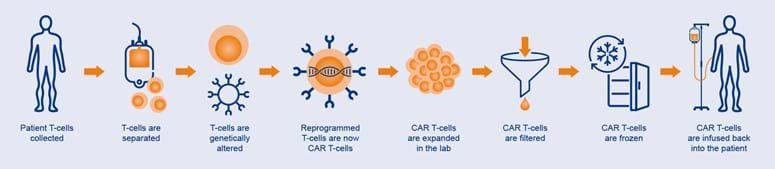
A CAR is a protein that is designed to recognize a specific antigen, which is a molecule on the surface of a cell. In the case of CAR T-cell therapy, the CAR is designed to recognize an antigen that is present on the surface of cancer cells. The process of CAR T-cell therapy typically involves several steps. First, T-cells are collected from the patient's blood. These cells are then modified in the laboratory to express the CAR. The modified T-cells are then expanded in number before being infused back into the patient. Once inside the patient, the CAR T-cells can recognize and attack cancer cells. Chimeric antigen receptor (CAR) T cell therapy has transformed hematological cancer treatment, with six therapies now approved by the FDA. FDA approvals of anti-CD19 CAR T cell products for both acute lymphoblastic leukemia and certain types of B cell lymphoma were the first approved gene therapies in the USA.
Establishing reliable methods of tracking CAR T-cell numbers is particularly important, not only in the estimation of effectiveness of CAR T cell therapy, but in terms of safety evaluation in post-infusion monitoring.
The History of CAR T-cell therapy
CAR T-cell therapy is a relatively new form of cancer treatment that has shown a lot of promise in recent years. Here are some of the key breakthroughs in CAR T-cell therapy since its inception:
- The origin: The idea of using T-cells to treat cancer dates back to the 1980s. However, it wasn't until the 1990s that the first clinical trials of T-cell therapy for cancer were conducted. These early trials focused on using T-cells that had been taken from the patient, grown in the laboratory, and then infused back into the patient.
- Genetic modification: In the early 2000s, researchers began to explore the idea of genetically modifying T-cells to improve their ability to recognize and attack cancer cells. The first CAR T-cell therapy trials were conducted in the mid-2000s, and these early trials showed promising results in treating certain types of blood cancers.
- First successful use of CAR T-cells: In 2010, a team of researchers at the University of Pennsylvania successfully used CAR T-cells to treat a patient with chronic lymphocytic leukemia (CLL). The patient went into complete remission and remained cancer-free for more than two years.
- FDA approval of CAR T-cell therapy: In 2017, the U.S. Food and Drug Administration (FDA) approved the first CAR T-cell therapy, called Kymriah, for the treatment of acute lymphoblastic leukemia (ALL).
- Expansion to other types of cancer: Since the initial success of CAR T-cell therapy in CLL and ALL, researchers have developed CAR T-cell therapies for other types of cancer, including multiple myeloma and non-Hodgkin's lymphoma.
- Development of "off-the-shelf" CAR T-cells: One of the challenges of CAR T-cell therapy is that it requires the patient's own T-cells to be harvested and modified, which can be time-consuming and expensive. In recent years, researchers have been working on developing "off-the-shelf" CAR T-cells that can be used in multiple patients without the need for individualized cell processing.
- Improved safety: One of the potential side effects of CAR T-cell therapy is cytokine release syndrome (CRS), a potentially life-threatening condition that can cause fever, low blood pressure, and organ damage. Researchers have been working on ways to make CAR T-cell therapy safer, including the use of medications to control CRS and the development of CAR T-cells that can be turned off if needed.
- Immune effector cell-associated neurotoxicity syndrome (ICANS) is a potentially serious side effect that can occur in patients undergoing chimeric antigen receptor (CAR) T-cell therapy. To address the risk of ICANS, improved safety measures have been implemented, such as the use of risk stratification algorithms, early intervention protocols, and the development of novel CAR T-cell designs that can reduce the incidence and severity of ICANS while maintaining efficacy against cancer cells. These advancements have helped to improve the safety profile of CAR T-cell therapy and reduce the occurrence of ICANS-related complications.
- Combination therapies: Researchers are also exploring the use of CAR T-cell therapy in combination with other cancer treatments, such as chemotherapy and checkpoint inhibitors, to improve outcomes for patients.
Testing the Success of CAR T-cell therapy
CAR T-cell expansion and persistence testing are important areas of research in the development of CAR T-cell therapy. Expansion refers to the process of growing large numbers of CAR T cells in the laboratory before infusing them back into the patient's body as well as the continued multiplication of the CAR T-cells post-infusion. This is necessary to ensure that there are enough CAR T cells to effectively target and destroy cancer cells. Persistence testing involves assessing the ability of CAR T-cells to recognize and attack cancer cells. By improving CAR T-cell expansion and persistence testing, researchers may be able to improve the efficacy and safety of CAR T-cell therapy in the future.
The Eurofins Viracor ExPeCTTM anti-CD19 (FMC63) CAR T-cell assay test is a diagnostic tool used to measure the expansion and persistence activity of chimeric antigen receptor (CAR) T-cells in patients undergoing CAR T-cell therapy for hematologic malignancies. For hpatients this assay provides early, actionable, and longitudinal data which may provide information on the expansion and persistence of genetically modified T cells used as therapeutic treatment for pre-B cell acute lymphoblastic leukemia (ALL) or B cell lymphomas. The Eurofins Viracor ExPeCT anti-CD19 (FMC63) CAR T-cell assay test is a valuable tool in the management of patients undergoing CAR T-cell therapy and can aid in optimizing treatment outcomes. This assay helps researchers and clinicians assess the effectiveness of CAR T-cell therapy and monitor patients for potential side effects. Currently, there are no direct CAR-T cell monitoring options commercially available, with B cell aplasia typically used as a surrogate marker for CD19-directed CAR-T cell efficacy.
The assay described here demonstrates excellent sensitivity, specificity, precision, and accuracy, providing a reliable means of monitoring anti-CD19 CAR-T cell expansion and in patients receiving any of the current approved therapies, as well as other CAR T-cell therapies under investigation using the FMC63 scFv. This has been validated in accordance with guidelines recommended by the New York State Department of Health, College of American Pathologists (CAP), and Clinical and Laboratory Standards Institute (CLSI) to establish the analytical specificity, linearity and dynamic range, analytical sensitivity (limit of detection and lower limit of quantification), intra- and inter-assay precision (reproducibility), and analytical accuracy of the test method. These tests are essential for ensuring the safety and efficacy of CAR T-cell therapy and advancing the development of this promising cancer treatment.
The Future of CAR T-cell therapy
CAR T-cell therapy is a rapidly advancing form of immunotherapy that has shown remarkable success in treating certain types of cancer. Although there are challenges associated with its widespread use, including high costs and potential side effects, ongoing research and development are improving the safety and efficacy of CAR T-cell therapy. With continued progress, CAR T-cell therapy has the potential to become a game-changing treatment for cancer, offering hope to patients and their families.