Know how to use results. Know how to treat.
ImmuKnow® is the only FDA-cleared, reimbursable assay designed to detect changes in immune cell function over time, giving you crucial insight for individualized patient management.
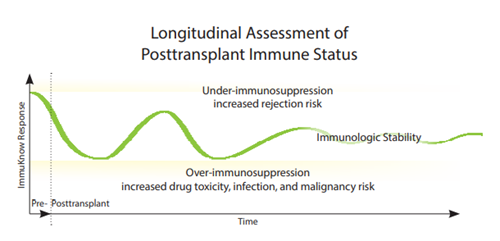
By identifying changes in CD4 cell ATP production1,2, the ImmuKnow® assay helps define a range of stable immune function for solid organ transplant patients (SOT).
Results that lie outside an individual patient’s range may help indicate an increased risk of infection or rejection and aid in the diagnosis and differentiation of SOT complications.
KNOW THE FACTS
Infection or Rejection?
Clinical utility has been well characterized and validated:
- Over 400,000 assays run
- 25 prospective and interventional studies in 1000+ transplant recipients
- More than 120 clinical studies
Global immune status monitoring has been used to:
- Identify kidney,4 liver,3 lung,5,6 and heart7 transplant patients with low immune response, which has the potential to lead to infection
- Define immunologic stability in various solid organ transplant patients7,8
- Longitudinally assess changes in transplant patients’ immune status, giving
clinicians an additional tool in treatment optimization decisions3,7-9
IMMUNE SYSTEM MONITORING
| Time | Interval |
| Pre-transplant | Test as needed |
| Months 1-6 | Test every 2 weeks |
| Months 7-12 | Test monthly |
| After Year 1 | Perform routine monitoring (at minimum, test quarterly) |
Resources
1 Augustine NH, Pasi BM, Hill HR. Comparison of ATP production in whole blood and lymphocyte proliferation in response to phytohemagglutinin. J Clin Lab Anal. 2007;21:265-270.
2 Sottong PR, Rosebrock JA, Britz JA, Kramer TR. Measurement of T-lymphocyte responses in whole-blood cultures using newly synthesized DNA and ATP. Clin Diagn Lab Immunol. 2000;7:307-311.
3 Cabrera R, Ararat M, Soldevila-Pico C, et al. Using an immune functional assay to differentiate acute cellular rejection from recurrent hepatitis C in liver transplant patients. Liver Transpl. 2009;15:216-222.
4 Sánchez-Velasco P, Rodrigo E, Valero R, et al. Intracellular ATP concentrations of CD4 cells in kidney transplant patients with and without infection. Clin Transplant. 2008;22:55-60.
5 Bhorade SM, Janata K, Vigneswaran WT, Alex CG, Garrity ER. Cylex ImmuKnow assay levels are lower in lung transplant recipients with infection. J Heart Lung Transplant. 2008;27:990-994.
6 Husain S, Raza K, Pilewski JM, et al. Experience with immune monitoring in lung transplant recipients: correlation of low immune function with infection. Transplantation. 2009;87:1852-1857.
7 Kiyosaki K, Kobashigawa J, Patel J, et al. The benefit of immune monitoring (Cylex): a review of 864 immune monitoring assays in heart transplantation. Presented at: The International Society for Heart and Lung Transplantation 29th Annual Meeting and Scientific Sessions; April 22-25, 2009; Paris, France; Abstract 511.
8 Knight RJ, Kerman RH, McKissick E, et al. Selective corticosteroid and calcineurin-inhibitor withdrawal after pancreas–kidney transplantation utilizing thymoglobulin induction and sirolimus maintenance therapy. Clin Transplant. 2008;22:645-650.
9 Kowalski RJ, Post DR, Mannon RB, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006;82:663-668.
10 Ravaioli M, Neri F, et al. Immunosuppression Modifications Based on an Immune Response Assay: Results of a Randomized, Controlled Trial. Transplantation. Epub 2015 March 9.
11 Kowalski RJ, Post DR, Mannon RB, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006;82:663-668.
Explore by Category





