Technologies
Genomic Profiling with PanCancerIQ™
Advancing the Future of Precision Oncology

We now offer comprehensive genomic profiling (CGP), using next-generation sequencing (NGS), for both FFPE tissue and liquid biopsies to support you precision oncology clinical development program(s).
Comprehensive Genomic Sequencing to Identify Somatic Alterations in Tumors (for both FFPE tissue and liquid/plasma biopsies)
Eurofins Viracor now offers a robust new next-generation sequencing (NGS) assay that enables comprehensive genomic profiling of tumor samples.
The Eurofins Viracor PanCancerIQ service uses the Illumina TruSight Oncology 500 (TSO500) system, combined with clinical interpretation through utilization of a knowledgebase developed by MD Anderson Cancer Center via a partnership with Philips.
The PanCancerIQ assay was designed to identify genomic alterations known to drive cancer growth: mutations, insertions and deletions (indels), copy number variations (CNV), and RNA-based gene fusions. In addition, it accurately measures key current immuno-oncology biomarkers: microsatellite instability (MSI), and tumor mutational burden (TMB).
The assay also identifies oncogenic driver events that predict response or resistance to treatments, helping clients accelerate their clinical trials.
Driven by science, the innovative, consultative approach of our Biopharma team enables clients to overcome obstacles and get faster results. Our new PanCancerIQ assay can help:
-
- Screen patients for enrollment.
- Stratify patient cohorts.
- Provide biomarker-driven trial optimization.
- Optimize clinical trial design and drug development decisions.
- Assess the efficacy of targeted anticancer therapies.
- Accelerate the implementation of precision oncology and guide the better use of targeted drugs.
Let our experts support your biomarker-driven clinical trial programs with the comprehensive NGS solution that provides proven utility. We can help you interrogate the oncogenome, and accurately identify clinically relevant genomic alterations, with unparalleled breadth and sensitivity, using our integrated genomic solutions to accelerate your oncology research and clinical development programs.
Variant Allele Frequency (VAF) Concordance of Matched FFPE and Plasma Samples from Patient Samples
Plasma-based assessment for mutations in ctDNA has become increasingly popular as a non-invasive and relatively quick way to assess for tumor specific mutations. In the graphic below we show the observed SNV (single nucleotide variant) concordance between matched tissue and plasma in 5 patients across 4 indications using the PanCancerIQ assay.
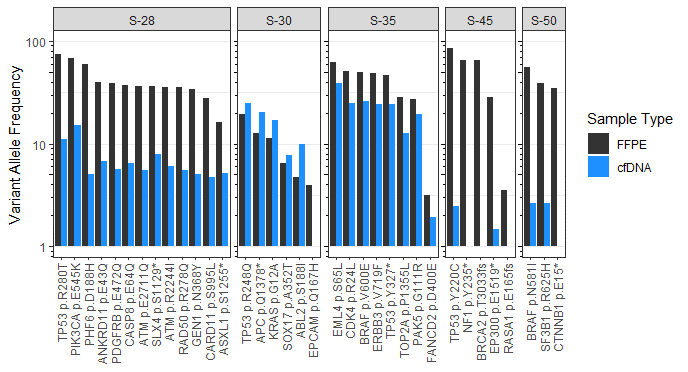
Each bar shows the variant allele frequency measured in FFPE (black) and ctDNA (blue) for the indicated mutation. Mutations identified align with expected variants described in the literature for each indication.
View or download our new brochure to earn more about our PanCancerIQ comprehensive genomic profiling service.

Eurofins Viracor BioPharma Offers:
-
- Rapid and accurate genetic analysis of clinical FFPE tissue
- Genomic services to support translational research, and clinical development of precision medicine
- Bioinformatics expertise for analysis, interpretation, and biomarker assay development
- Extensive global testing network
- Improved limits of detection and coverage depth
- Comprehensive genomic sequencing now validated for liquid biopsies (plasma)

